New COVID-19 treatment to replace Paxrovid?

As Gilead grew into a big pharmaceutical company 20 years ago by inventing Tamiflu, the mega-hit influenza virus treatment, in South Korea there is a promising bio-tech company that can grow into the next big thing with their own ground breaking technology to treat corona virus.
As the introduction of Penicillin heralded the era of the anti-bacterial age, the potential magic bullet for corona can be recreated as a universal anti-virus drug.
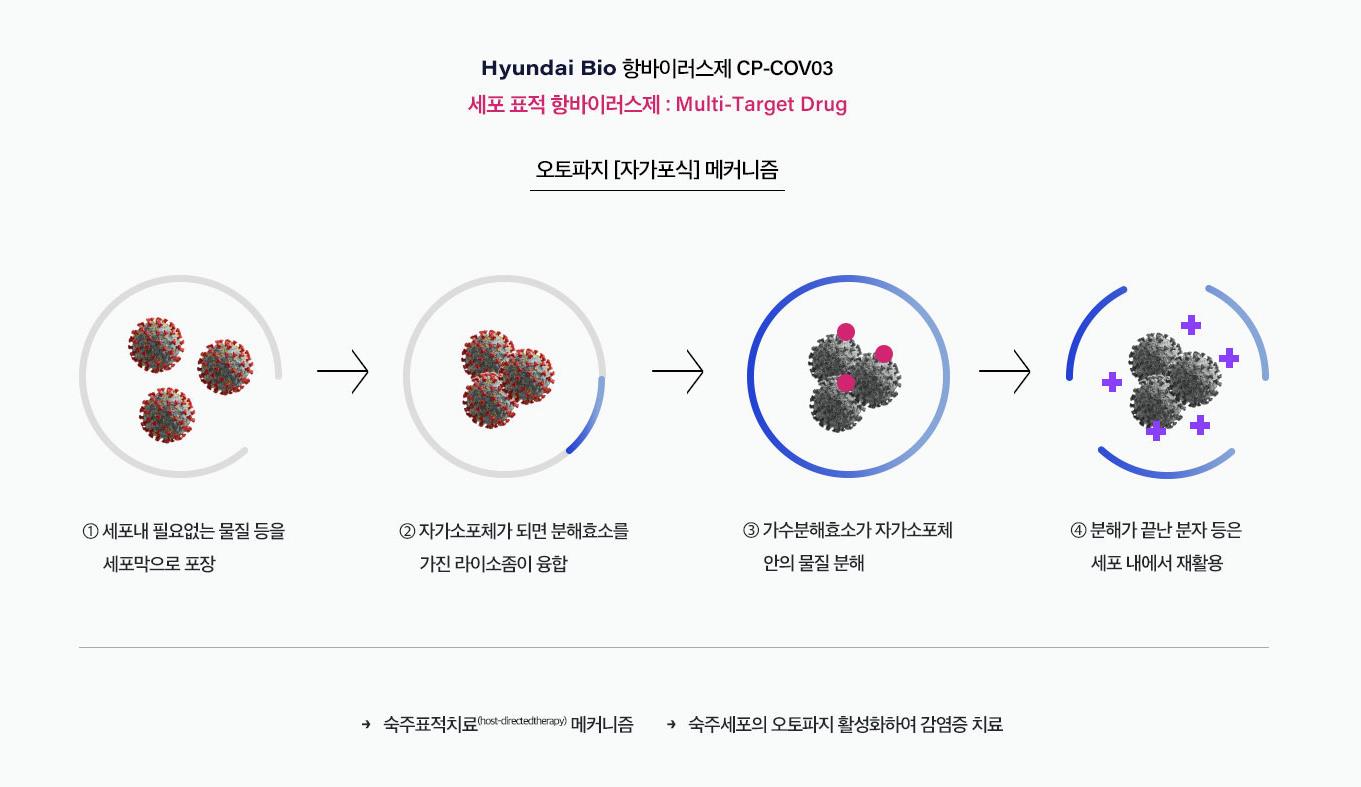
Hyundai Bio Science is the company. They are very likely to contribute to ending the Corona pandemic by successfully developing an anti viral medicine.
Their technology doesn’t only work for corona virus but for almost all other virus-related diseases.
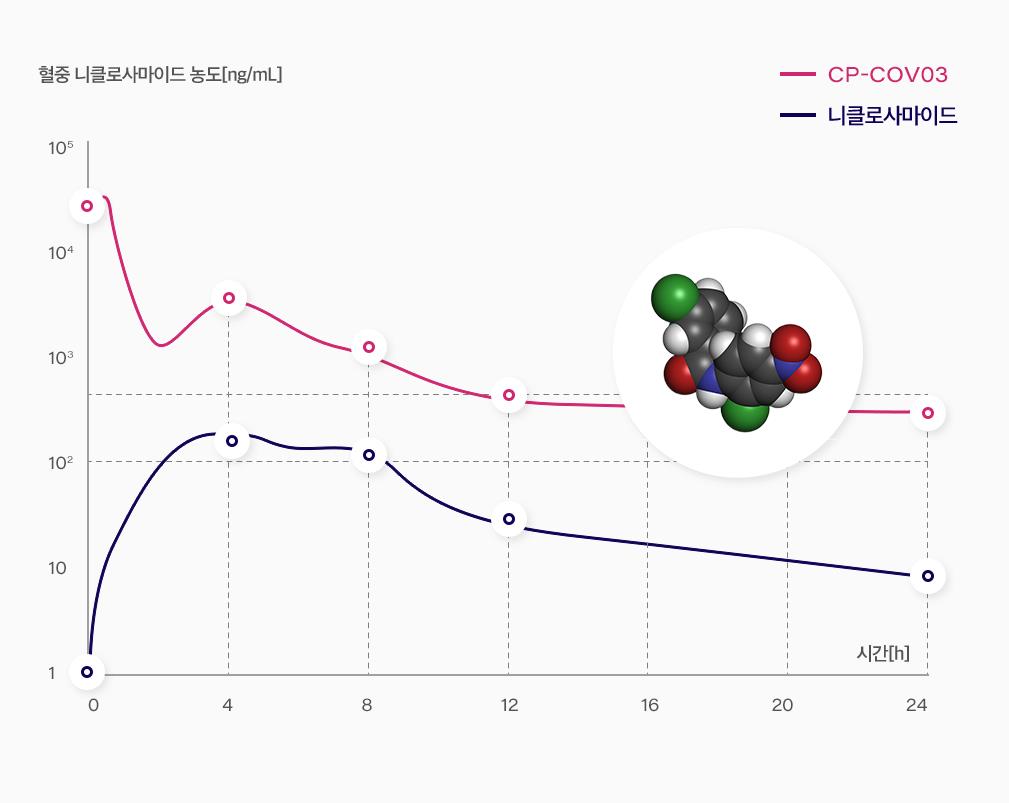
Niclosamide, originally developed as a anthelmintic drug, is already well-known as one of the most promising candidates against SARS-COV-2.
However, its low bioavailability and short half-life in plasma concentration made it difficult to be repurposed.
Hyundai Bio Science has proved that they have the cutting-edge technology to improve the bioavailability and short-half life of Niclosamide in the human body by successfully completing several pre-clinical tests and a phase 1 clinical trial, something which global Big Pharmaceutical companies have failed to do in the last 60 years.
Their phase 2 clinical trial is in the final stages and the results will be announced soon.
The Data monitoring committee, called the DSMB, of the Korean Food and Drug Safety Department has confirmed the safety and efficacy of the drug.
The company briefly announced the provisional result of the PK test from the phase 2 clinical trial and will apply for EUA from the Korean Food and Drug Safety Department with the data from the phase 2 test.
Many research institutes are trying to repurpose Niclosamide for use against other diseases.
1) [Covid19] - Charité Research Organisation GmbH Berlin, Germany (Covid19)
2) [Colon Cancer] - Duke University, Durham, North Carolina, US
3) [Metastatic Prostate Carcinoma] - University of California Davis Comprehensive Cancer Center, Sacramento, CA, US
[Recurrent Prostate Carcinoma] - University of California Davis Comprehensive Cancer Center, Sacramento, CA, US
[Stage IV Prostate Cancer] - University of California Davis Comprehensive Cancer Center, Sacramento, CA, US
4) [COVID-19] - Tufts Medical Center Boston, Massachusetts, US
5) [COVID-19] - Central Alabama Research, Birmingham, Alabama, United States
6) [COVID-19] - Midland Florida Clinical Research Center, LLC
7) [COVID-19] - DeLand, Florida, US
8) [COVID-19] - New Generation Medical Research, US
9) [COVID-19] - Hialeah, Florida, US
10) [COVID-19] - Helen Keller Hospital Sheffield, Alabama, US
11) [COVID-19] - DanTrials ApS Copenhagen, Denmark
12) [COVID-19] - National University Hospital, Singapore, Singapore
Renowned institutes have already proved the extensive efficacy of Niclosamide through in-vitro tests.
(MULTI TARGET DRUG NICOLOSAMIDE)
1. [MERS-Cov] - (Gasen et. all. Nat Commun[2019] 10 : 5770),
2. [SARS-CoV-1] - (Wu et al Artibicnob Agents Chemotehr[2004]48,2693-6) ,
3. [SARS-CoV-2] - (Jeon et al Antimicnob Agents Chemother, [2020]64(7):e00819-20),
[ HIV ] - (Jeon et al Antimicnob Agents Chemother, [2020]64(7):e00819-20),
4. [Zika Virus] - (Simeonov et. all. [2016] Nat, Med, 22, 1101-7),
5. [Dengue virus2] - (Li et. all. Cell Res [2017] 26. 1046-64),
[West nie virus] - (Li et. all. Cell Res [2017] 26. 1046-64),
[Yellow feer virus] - (Li et. all. Cell Res [2017] 26. 1046-64),
[Japanese enc ephalthis virus] - (Li et. all. Cell Res [2017] 26. 1046-64),
6. [hepatitis E virus HEV] - (Edwards et. all. J Med, Chem [2011]54, 8670-80)
[hepatitis C virus HEV] - (Edwards et. all. J Med, Chem [2011]54, 8670-80)
7. [Ebola virus] - (Madrid et al. [2015]ACS infect dis 1.317-26)
8. [Influenza Virus] - (Jurgelt et al PLoS apthog [2012]8[1:0]e 1002976)
[Herpes Virus 1(45w1)] - (Jurgelt et al PLoS apthog [2012]8[1:0]e 1002976)
[Human Rhino Virus] - (Jurgelt et al PLoS apthog [2012]8[1:0]e 1002976)
[Coxsackievirus] - (Jurgelt et al PLoS apthog [2012]8[1:0]e 1002976)
NBC introduced Hyundai Bio Science
Recently updated
[May - 2022] phase 2 clinical trial of CP-COV03 for COVID-19 patients has begun.
[July - 2022] DSMB(Data monitoring committee)perfectly recommend continue phase 2 clinical trial based on data which positive drug effect and no side effect
[Sep - 2022] patient for PK(Pharmacodnamics) recruiting completed
[Nov- 2022] phase 2 clinical trial recruiting completed
[Dec - 2022] the company announced remarkable PK data preview (have reached IC50 and IC100)
[Jan - 2023] Hyundai Bio Science will announce PK(Pharmacodnamics) Topline DATA (ASAP)

'주식·크립토·경제' 카테고리의 다른 글
| 소니드, 2차전지 테마로 상승 보여줄까? (0) | 2023.05.18 |
|---|---|
| 5월 12일자 현대바이오 관련 언론 보도 (0) | 2023.05.14 |
| ROE 유보율 등 재무지표를 포함한 조건검색식 (0) | 2023.03.29 |
| EBS에서 본 현대바이오의 범용 항바이러스제 (0) | 2023.03.15 |
| 국산 코로나19 경구용 치료제, 현대바이오만 남았나 (0) | 2022.12.28 |
| 주식 일목균형표 양운 출현 조건 검색식 (0) | 2022.12.28 |
| 2차 전지 리튬 관련주 지엔원에너지 (0) | 2022.12.28 |
| 2차 전지 관련주 동화기업, 무상증자만 해주면 (0) | 2022.12.27 |
